"ISO 20695: Enteral feeding systems — Design and testing"
ISO 20695 will specify the requirements for all the enteral feeding devices: administration sets, feeding tubes, extension lines, syringes and accessories.
Indeed, ISO 20695 is the revision of the existing EN 1615/EN 1618* standards, converting into an international standard and incorporating the small-bore connectors’ requirements of ISO 80369 (Part 1 and 3).
This ISO 20695 standard is still under development within the CEN/TC205 /WG16 group. Vygon is one of the expert members of this working group.
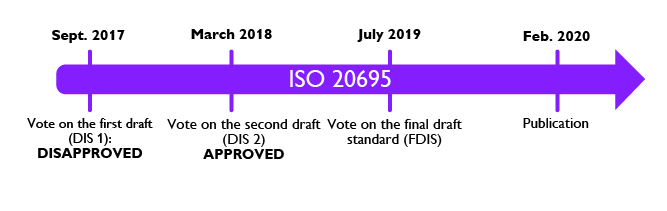
Main steps in the creation of ISO 20695
The first draft of ISO 20695 was disapproved at the international level in September 2017.
One of the main reasons is that the so-called “Low Dose Tip” (LDT) syringe had been incorporated into the ISO 20695 standard, while its ability to reliably increase dosage accuracy was not demonstrated:
- Experimentations have shown that an overdose is clearly possible, if fluid is present in the moat of the LDT syringe.
- Computational Fluid Dynamics simulations (taking into account physical parameters such as gravity, viscosity, rotational speed, etc.) have shown that this overdose was 0.120 ml, which is roughly equivalent to the conventional ENFit syringe.
The second draft of ISO 20695 was approved at the international level in March 2019.
The main reasons are:
- The Low Dose Tip syringe (LDT) is recognized for not reliably increasing dose accuracy.
- The LDT has been removed from the normative (mandatory) requirements and is now included as information only!
- The LDT is now called « enteral alternative syringe tip » as it can’t ensure accuracy of low dosage.
Here the extract of the second ISO 20695 draft – Annex K relative to the « enteral alternative syringe tip » (previously called “Low Dose Tip syringe”) :
- “CEN/TC 205/WG 16 has decided to propose it for information only as test results lead to contradictory conclusions on dose accuracy with this alternative enteral syringe tip.”
- “CEN/TC 205/WG 16 has not reached consensus regarding the ability of the alternative enteral syringe tip […] to reliably increase dose accuracy. In addition, the design of this alternative enteral syringe tip may not ensure that the dose accuracy for enteral syringes is met; specific user information and education should accompany this alternative enteral syringe based upon the manufacturer’s risk management file”
See the professionals’ concerns for infants on the inaccuracy risk of the ENFit and LDT syringes.